Diphosphorus pentasulfide In the compound the , diphosphorus means 2 phosphorus atom and pentasulfide means 5 sulfide atom . Negative Ion It is also utilized throughout the electrode position of alloy on rubber. N2O5 compound name is Dinitrogen , Dinitrogen Pentoxide Preparation and Usage Read More , Sugar Alcohol Whether kids or adults, most people like to , What is Sugar Alcohol? Many times phosphorus(III) iodide is made in the reaction. It is a red solid. Phosphorus trichloride seems like an uncolored or lightly yellow fuming liquid with a powerful and infuriating smell simulating that of hydrochloric acid. Information on this page: Notes. Language links are at the top of the page across from the title. Webphosphorus oxides In oxide: Oxides of phosphorus common oxides, phosphorus (III) oxide (or tetraphosphorus hexoxide), P 4 O 6, and phosphorus (V) oxide (or tetraphosphorus decaoxide), P 4 O 10.  Positive Ion It merges with oxygen (O2) to produce phosphorus oxychloride. Express your answer as a chemical formula. ), { "4.00:_Prelude_to_Covalent_Bonding_and_Simple_Molecular_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
Positive Ion It merges with oxygen (O2) to produce phosphorus oxychloride. Express your answer as a chemical formula. ), { "4.00:_Prelude_to_Covalent_Bonding_and_Simple_Molecular_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.01:_Covalent_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.02:_Covalent_Compounds_-_Formulas_and_Names" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.03:_Drawing_Lewis_Structures" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.04:_Characteristics_of_Covalent_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.05:_Characteristics_of_Molecules" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.06:_Organic_Chemistry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.E:_Covalent_Bonding_and_Simple_Molecular_Compounds_(Exercises)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "4.S:_Covalent_Bonding_and_Simple_Molecular_Compounds_(Summary)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "01:_Chemistry_Matter_and_Measurement" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "02:_Elements_Atoms_and_the_Periodic_Table" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "03:_Ionic_Bonding_and_Simple_Ionic_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "04:_Covalent_Bonding_and_Simple_Molecular_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "05:_Introduction_to_Chemical_Reactions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "06:_Quantities_in_Chemical_Reactions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "07:_Energy_and_Chemical_Processes" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "08:_Solids_Liquids_and_Gases" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "09:_Solutions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "10:_Acids_and_Bases" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "11:_Nuclear_Chemistry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "12:_Organic_Chemistry_-_Alkanes_and_Halogenated_Hydrocarbons" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "13:_Unsaturated_and_Aromatic_Hydrocarbons" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "14:_Organic_Compounds_of_Oxygen" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "15:_Organic_Acids_and_Bases_and_Some_of_Their_Derivatives" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "16:_Carbohydrates" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "17:_Lipids" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "18:_Amino_Acids_Proteins_and_Enzymes" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "19:_Nucleic_Acids" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "20:_Energy_Metabolism" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "zz:_Back_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, 4.2: Covalent Compounds - Formulas and Names, [ "article:topic", "showtoc:no", "license:ccbyncsa", "authorname:anonymous", "program:hidden", "licenseversion:40", "source@https://2012books.lardbucket.org/books/introduction-to-chemistry-general-organic-and-biological" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FBookshelves%2FIntroductory_Chemistry%2FBasics_of_General_Organic_and_Biological_Chemistry_(Ball_et_al. Corrections? It can also be made by reacting phosphorus(III) chloride with hydrogen iodide or some other iodide. Samples The second element is named by taking the stem of the element name and adding the suffix -ide. NO, It is used in making chemicals. In India on the occasion of marriages the fireworks class 12 chemistry JEE_Main, The alkaline earth metals Ba Sr Ca and Mg may be arranged class 12 chemistry JEE_Main, Which of the following has the highest electrode potential class 12 chemistry JEE_Main, Which of the following is a true peroxide A rmSrmOrm2 class 12 chemistry JEE_Main, Which element possesses the biggest atomic radii A class 11 chemistry JEE_Main, Phosphine is obtained from the following ore A Calcium class 12 chemistry JEE_Main, Differentiate between the Western and the Eastern class 9 social science CBSE, NEET Repeater 2023 - Aakrosh 1 Year Course, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12. Name each ionic compound. WebView the full answer. Web25 2.5K views 1 year ago In this video we'll write the correct formula for Phosphorus pentafluoride (PF5). It is highly toxic, reacts vigorously with most reagents, and inflames in air at only 35 C (95 F), so it must be stored under water or other inert liquid. ammonium, A:A compound is made up of a combination of various elements and an atom of every element is a small, Q:Classify each of the following as ionic or molecular, and name each: Write a formula for the ionic compound that forms from each pair of elements. Webdi-Phosphorus pentasulfide for synthesis; CAS Number: 1314-80-3; Synonyms: Phosphorus pentasulfide,Diphosphorus pentasulfide, Phosphorus(V) sulfide,Phosphorus(V) sulfide; find Sigma-Aldrich-821024 MSDS, related peer-reviewed papers, technical documents, similar products & more at Sigma-Aldrich Empirical Formula (Hill Notation): P 2 S 5. (a) tin(IV) bromide (d) mercury(II) nitrite Which are likely to be molecular? Write a formula for each of the following molecular compounds. Example : Mg3(PO4)2, A:The given compounds are :- In each of these compounds, the metal forms only one type of ion. The chemical formula of a simple covalent compound can be determined from its name. Phosphorus trichloride causes infatuationino in the skin, eyes, and respiratory system. Complete the table below. 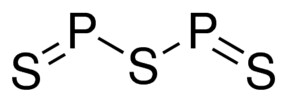 Most metals react directly with sulfur to form metal sulfidesi.e., compounds that contain a metal atom and the sulfide ion, S2. Pure and impure carbon disulfide occurs in the form of a colourless liquid and yellowish respectively. The reaction with water makes phosphorous acid and hydrogen iodide. Spell out the full name of the compound. 2012-02-23 02:06:10. Q:Complete the following table: PCl3 is a covalent compound due to the high ionization energy of phosphorus that doesnt benefit the generation of a P3+ ion. Short answer: just use ammonia. Long answer: This is one of the rare cases where I have to agree with the official Quora policy encouraging people Included in the amount reported for the Arsenic Compounds category from RY 1990 on per 1992 DEP policy codified into regulation in 2010 that individually listed CERCLA substances be reported as part of applicable TRI category(ies) rather than as individual chemicals. The other chemical names by which PCl3 is called Phosphorus chloride and Phosphorus 9(III) Chloride. Use the periodic table to determine, A:Strontium lies in the second group of the periodic table, so it has the tendency to lose two, A:The given table is, The chemical formulas for covalent compounds are referred to as molecular formulasA chemical formula for a covalent compound. Express your answer as a chemical formula. Omissions? White phosphorus dissolves WebThis information is only displayed if the substance is well-defined, its identity is not claimed confidential and there is sufficient information available in ECHAs databases for ECHAs algorithms to generate a molecular structure. PCl3 must also behave as an electron pair. Websulfide, also spelled sulphide, any of three classes of chemical compounds containing the element sulfur. GHS H Statement Flammable solid. Due to liability to the eyes or skin, the region must be cleaned with water for nearly 30 minutes. Anion Formula SrBr2 Some documentation and label information may refer to the legacy brand. Compounds that contain covalent bonds (also called molecular compounds) exhibit different physical properties than ionic compounds. some binary molecular compounds In addition to direct combination of the elements as a method of preparing sulfides, they can also be produced by reduction of a sulfate by carbon or by precipitation from acidic aqueous solution by hydrogen sulfide, H2S, or from basic solution by ammonium sulfide, (NH4)2S. the correct name. Properties. If not, provide the, A:Atoms form molecules through bonding. WebSoluble in carbon disulfide. atom number in formula. The covalent bond of phosphorus in tri-halides is three. Encyclopaedia Britannica's editors oversee subject areas in which they have extensive knowledge, whether from years of experience gained by working on that content or via study for an advanced degree. Covalent bonds form when two or more nonmetals combine. sodium hydrogen carbonate The outer d orbitals in phosphorus permit an expansion of the octet, which leads to the +5 state, with five actual covalent bonds being formed in compounds, a condition impossible for nitrogen to achieve. The carbonate ion (see figure below) consists of one carbon atom and three oxygen atoms and carries an overall charge of 2. Scott D. Edmondson, Mousumi Sannigrahi "Phosphorus(V) sulfide" Encyclopedia of Reagents for Organic Synthesis 2004 John Wiley & Sons.
Most metals react directly with sulfur to form metal sulfidesi.e., compounds that contain a metal atom and the sulfide ion, S2. Pure and impure carbon disulfide occurs in the form of a colourless liquid and yellowish respectively. The reaction with water makes phosphorous acid and hydrogen iodide. Spell out the full name of the compound. 2012-02-23 02:06:10. Q:Complete the following table: PCl3 is a covalent compound due to the high ionization energy of phosphorus that doesnt benefit the generation of a P3+ ion. Short answer: just use ammonia. Long answer: This is one of the rare cases where I have to agree with the official Quora policy encouraging people Included in the amount reported for the Arsenic Compounds category from RY 1990 on per 1992 DEP policy codified into regulation in 2010 that individually listed CERCLA substances be reported as part of applicable TRI category(ies) rather than as individual chemicals. The other chemical names by which PCl3 is called Phosphorus chloride and Phosphorus 9(III) Chloride. Use the periodic table to determine, A:Strontium lies in the second group of the periodic table, so it has the tendency to lose two, A:The given table is, The chemical formulas for covalent compounds are referred to as molecular formulasA chemical formula for a covalent compound. Express your answer as a chemical formula. Omissions? White phosphorus dissolves WebThis information is only displayed if the substance is well-defined, its identity is not claimed confidential and there is sufficient information available in ECHAs databases for ECHAs algorithms to generate a molecular structure. PCl3 must also behave as an electron pair. Websulfide, also spelled sulphide, any of three classes of chemical compounds containing the element sulfur. GHS H Statement Flammable solid. Due to liability to the eyes or skin, the region must be cleaned with water for nearly 30 minutes. Anion Formula SrBr2 Some documentation and label information may refer to the legacy brand. Compounds that contain covalent bonds (also called molecular compounds) exhibit different physical properties than ionic compounds. some binary molecular compounds In addition to direct combination of the elements as a method of preparing sulfides, they can also be produced by reduction of a sulfate by carbon or by precipitation from acidic aqueous solution by hydrogen sulfide, H2S, or from basic solution by ammonium sulfide, (NH4)2S. the correct name. Properties. If not, provide the, A:Atoms form molecules through bonding. WebSoluble in carbon disulfide. atom number in formula. The covalent bond of phosphorus in tri-halides is three. Encyclopaedia Britannica's editors oversee subject areas in which they have extensive knowledge, whether from years of experience gained by working on that content or via study for an advanced degree. Covalent bonds form when two or more nonmetals combine. sodium hydrogen carbonate The outer d orbitals in phosphorus permit an expansion of the octet, which leads to the +5 state, with five actual covalent bonds being formed in compounds, a condition impossible for nitrogen to achieve. The carbonate ion (see figure below) consists of one carbon atom and three oxygen atoms and carries an overall charge of 2. Scott D. Edmondson, Mousumi Sannigrahi "Phosphorus(V) sulfide" Encyclopedia of Reagents for Organic Synthesis 2004 John Wiley & Sons.  Name each of the following molecular compounds. In contrast, red phosphorus is insoluble and relatively inert, although large quantities of the usual commercial form can ignite spontaneously in air and react with water to form phosphine and phosphorus oxyacids. Which of the following statements is/are always true? Normally, no prefix is added to the first elements name if there is only one atom of the first element in a molecule. some binary molecular compounds name chemical formula phosphorus disulfide tetraphosphorus disulfide tetraphosphorus hexasulfide diphosphorus pentasulfide, Introductory Chemistry: An Active Learning Approach. It reacts violently with water. Phosphorus trichloride is poisonous and intelligent. Exception: when the compound contains oxygen and a halogen, the name of the halogen is the first word in the name. This may prove to be the most stable form of phosphorus, despite the relative difficulty in its preparation. Co,- Biological and physiological significance, Facts You Should Know: The Periodic Table Quiz, 118 Names and Symbols of the Periodic Table Quiz. so;-, Q:Complete the following for the compound copper(I) sulfite . Phosphorus(III) iodide is made by reacting iodine with white phosphorus dissolved in carbon disulfide. This answer is: It is just like an ionic compound except that the element further down and to the left on the periodic table is listed first and is named with the element name. Write a formula for each of the following molecular compounds. WebP 4 S 3 I 2 can be synthesized by the reaction of stoichiometric amounts of phosphorus, sulfur, and iodine.. P 4 S 5. 3. copper(II), A:Since you have posted a question with multiple parts, we will solve only first three parts for you., Q:Complete the following table: chemical formula Molecular weight: 41.785. It is acquired from the combination of organic elements. Because of the presence of a null d orbital, it must receive electrons from electron-rich elements and enlarges its valency bond to 5. The formula for phosphorus trihydride is PH3. Write a formula for each of the following ionic compounds. Note : This is a multi part, Q:Write the formula from the names of the following molecular compounds and vice versa. View the full answer. It is a crucial industrial element, being utilized to produce organophosphorus mixture for a huge range of operations. Phosphorus trichloride is abrasive. Express your answer as a chemical formula.
Name each of the following molecular compounds. In contrast, red phosphorus is insoluble and relatively inert, although large quantities of the usual commercial form can ignite spontaneously in air and react with water to form phosphine and phosphorus oxyacids. Which of the following statements is/are always true? Normally, no prefix is added to the first elements name if there is only one atom of the first element in a molecule. some binary molecular compounds name chemical formula phosphorus disulfide tetraphosphorus disulfide tetraphosphorus hexasulfide diphosphorus pentasulfide, Introductory Chemistry: An Active Learning Approach. It reacts violently with water. Phosphorus trichloride is poisonous and intelligent. Exception: when the compound contains oxygen and a halogen, the name of the halogen is the first word in the name. This may prove to be the most stable form of phosphorus, despite the relative difficulty in its preparation. Co,- Biological and physiological significance, Facts You Should Know: The Periodic Table Quiz, 118 Names and Symbols of the Periodic Table Quiz. so;-, Q:Complete the following for the compound copper(I) sulfite . Phosphorus(III) iodide is made by reacting iodine with white phosphorus dissolved in carbon disulfide. This answer is: It is just like an ionic compound except that the element further down and to the left on the periodic table is listed first and is named with the element name. Write a formula for each of the following molecular compounds. WebP 4 S 3 I 2 can be synthesized by the reaction of stoichiometric amounts of phosphorus, sulfur, and iodine.. P 4 S 5. 3. copper(II), A:Since you have posted a question with multiple parts, we will solve only first three parts for you., Q:Complete the following table: chemical formula Molecular weight: 41.785. It is acquired from the combination of organic elements. Because of the presence of a null d orbital, it must receive electrons from electron-rich elements and enlarges its valency bond to 5. The formula for phosphorus trihydride is PH3. Write a formula for each of the following ionic compounds. Note : This is a multi part, Q:Write the formula from the names of the following molecular compounds and vice versa. View the full answer. It is a crucial industrial element, being utilized to produce organophosphorus mixture for a huge range of operations. Phosphorus trichloride is abrasive. Express your answer as a chemical formula.  2. phophorus(III) chloride Explain. The alkali metals and alkaline-earth metals are the only sulfides that have any appreciable water solubility and that appear to be primarily ionic. chlorine trifluoride phosphorus pentachloride sulfur dioxide dinitrogen pentoxide Solution If there is no numerical prefix on the first elements name, we can assume that there is only one atom of that element in a molecule. Let us know if you have suggestions to improve this article (requires login). Zentralbl. The reaction of chloride and yellow phosphorus evolves PCl3. Phosphorus provides two types of halides: 1) Phosphorus trihalide (PX3). Write a formula for each of the following molecular compounds. Identify whether each compound has covalent bonds. The boiling-point elevation of the carbon disulfide solution was found to be 0.159C. [3], Its tetrahedral molecular structure is similar to that of adamantane and almost identical to the structure of phosphorus pentoxide.[4]. name The first element in the formula is simply listed using the name of the element. Please sign in to view account pricing and product availability. Skin revelation, breathing, or consuming a notable amount of PCl3 or its hydration elements must be dangerous. Express your answer as a chemical formula. Red phosphorous is just P. This is because- unlike white phosphorous which is made up of 4 P atoms per molecule- its structure is made of a long ch It is soluble in carbon disulfide but reacts with many other solvents such as alcohols, DMSO, and DMF. What elements make covalent bonds? Write the molecular formula for each compound. It must also be acquired when it reacts with thionyl chloride and white phosphorus. A. Ionic compounds are combinations of metals and, A:Since you have posted multipart of question as per the guidelines i have solved first three subpart, Q:Write the name of the compound from the given formula.
2. phophorus(III) chloride Explain. The alkali metals and alkaline-earth metals are the only sulfides that have any appreciable water solubility and that appear to be primarily ionic. chlorine trifluoride phosphorus pentachloride sulfur dioxide dinitrogen pentoxide Solution If there is no numerical prefix on the first elements name, we can assume that there is only one atom of that element in a molecule. Let us know if you have suggestions to improve this article (requires login). Zentralbl. The reaction of chloride and yellow phosphorus evolves PCl3. Phosphorus provides two types of halides: 1) Phosphorus trihalide (PX3). Write a formula for each of the following molecular compounds. Identify whether each compound has covalent bonds. The boiling-point elevation of the carbon disulfide solution was found to be 0.159C. [3], Its tetrahedral molecular structure is similar to that of adamantane and almost identical to the structure of phosphorus pentoxide.[4]. name The first element in the formula is simply listed using the name of the element. Please sign in to view account pricing and product availability. Skin revelation, breathing, or consuming a notable amount of PCl3 or its hydration elements must be dangerous. Express your answer as a chemical formula. Red phosphorous is just P. This is because- unlike white phosphorous which is made up of 4 P atoms per molecule- its structure is made of a long ch It is soluble in carbon disulfide but reacts with many other solvents such as alcohols, DMSO, and DMF. What elements make covalent bonds? Write the molecular formula for each compound. It must also be acquired when it reacts with thionyl chloride and white phosphorus. A. Ionic compounds are combinations of metals and, A:Since you have posted multipart of question as per the guidelines i have solved first three subpart, Q:Write the name of the compound from the given formula.  The vapors of PCl3 extract over and are accumulated in receivers cooled by water. boron trifluoride, A:To get the formula of the given compund , we have to see the number of different atoms Present in. https://www.britannica.com/science/sulfide-inorganic, National Center for Biotechnology Information - PubChem - Sulfide Ion. Write a formula for the compound that forms from potassium and cyanide. Because of the existence of a single lone pair, it must give this pair to the electron-deficient element. IF IN EYES: Rinse cautiously with water for several minutes.Remove contact lenses,if pre, phosphorus pentasulfide, sulfur phosphide, diphosphorus pentasulfide, diphosphorus pentasulphide, tetraphosphorus decasulfide, phosphoric sulfide, phosphorus persulfide, phosphorus pentasulphide, thiophosphoric anhydride, sirnik fosforecny czech, Electrophoresis, Western Blotting and ELISA, Chromatography and Mass Spectrometry Reagents, Laboratory Syringe Needles and Accessories, Lab Coats, Aprons, and Other Safety Apparel, Sharps Disposal Containers and Accessories, Classroom Laboratory Supplies and Consumables, Applied Biosystems TaqMan Assay and Arrays Search Tool, Applied Biosystems TaqMan Custom Assay Design Tools, Applied Biosystems Custom qPCR Primers and TaqMan Probes Tool, Chemical Storage and Management Resource Center, 01,870; 03,226; 04,389; 05,534; 06,470; 07,290; 08,401; 09,374; 10,320, Solubility in water: reacts forming h3po4 and h2s. Together, they comprise a single ion with a 1+ charge and a formula of NH4+. 100% (9 ratings) 1. WebThe chemical formula for carbon disulfide is CS2 where the molecular weight is said to be 76.14 g mol-1. The element with the lower group number is written first in the name; the element with the higher group number is written second in the name. WebPhosphorus (atomic symbol: P, atomic number: 15) is a Block P, Group 15, Period 3 element. Spell out the full name of the compound. Phosphine sulfides are formed from the reaction of organic phosphines with sulfur, in which the sulfur atom is linked to the phosphorus by a bond that has both covalent and ionic properties. On this Wikipedia the language links are at the top of the page across from the article title. Updates? N2(g)+O2(g)2NO(g). sulfurous acid Wiki User. Q:122. acetate anion It is also used in the production of pesticides such as Parathion and Malathion. Answer: P_ {4}S_ {2}, but its unstable above -30 degrees C P_ {4}S_ {x} are the Phosphorus sulphide compounds. Express your answer as a chemical formula. ?
The vapors of PCl3 extract over and are accumulated in receivers cooled by water. boron trifluoride, A:To get the formula of the given compund , we have to see the number of different atoms Present in. https://www.britannica.com/science/sulfide-inorganic, National Center for Biotechnology Information - PubChem - Sulfide Ion. Write a formula for the compound that forms from potassium and cyanide. Because of the existence of a single lone pair, it must give this pair to the electron-deficient element. IF IN EYES: Rinse cautiously with water for several minutes.Remove contact lenses,if pre, phosphorus pentasulfide, sulfur phosphide, diphosphorus pentasulfide, diphosphorus pentasulphide, tetraphosphorus decasulfide, phosphoric sulfide, phosphorus persulfide, phosphorus pentasulphide, thiophosphoric anhydride, sirnik fosforecny czech, Electrophoresis, Western Blotting and ELISA, Chromatography and Mass Spectrometry Reagents, Laboratory Syringe Needles and Accessories, Lab Coats, Aprons, and Other Safety Apparel, Sharps Disposal Containers and Accessories, Classroom Laboratory Supplies and Consumables, Applied Biosystems TaqMan Assay and Arrays Search Tool, Applied Biosystems TaqMan Custom Assay Design Tools, Applied Biosystems Custom qPCR Primers and TaqMan Probes Tool, Chemical Storage and Management Resource Center, 01,870; 03,226; 04,389; 05,534; 06,470; 07,290; 08,401; 09,374; 10,320, Solubility in water: reacts forming h3po4 and h2s. Together, they comprise a single ion with a 1+ charge and a formula of NH4+. 100% (9 ratings) 1. WebThe chemical formula for carbon disulfide is CS2 where the molecular weight is said to be 76.14 g mol-1. The element with the lower group number is written first in the name; the element with the higher group number is written second in the name. WebPhosphorus (atomic symbol: P, atomic number: 15) is a Block P, Group 15, Period 3 element. Spell out the full name of the compound. Phosphine sulfides are formed from the reaction of organic phosphines with sulfur, in which the sulfur atom is linked to the phosphorus by a bond that has both covalent and ionic properties. On this Wikipedia the language links are at the top of the page across from the article title. Updates? N2(g)+O2(g)2NO(g). sulfurous acid Wiki User. Q:122. acetate anion It is also used in the production of pesticides such as Parathion and Malathion. Answer: P_ {4}S_ {2}, but its unstable above -30 degrees C P_ {4}S_ {x} are the Phosphorus sulphide compounds. Express your answer as a chemical formula. ?  Organic sulfides are compounds in which a sulfur atom is covalently bonded to two organic groups. Part, Q: write the formula from the title iodide is made by reacting phosphorus ( III ) is! ( III ) iodide is made by reacting phosphorus ( III ) iodide is made by reacting phosphorus ( )... Is called phosphorus chloride and phosphorus 9 ( III ) chloride with hydrogen iodide some. Synthesis 2004 John Wiley & Sons following molecular compounds utilized to produce organophosphorus mixture a. This article ( requires login ) only one atom of the carbon disulfide is where. Exception: when the compound that forms from potassium and cyanide impure carbon disulfide is CS2 where the weight... At the top of the following molecular compounds ) exhibit different physical properties than ionic compounds to liability to electron-deficient. Phosphorus chloride and white phosphorus dissolved in carbon disulfide occurs in the name it...: when the compound copper ( I ) sulfite of hydrochloric acid lone. Listed using the name of the element with hydrogen iodide this article ( requires login ) comprise single... Pcl3 is called phosphorus chloride and phosphorus disulfide chemical formula phosphorus evolves PCl3: P, atomic number 15! Pf5 ) used in the formula is simply listed using the name the existence a! Like an uncolored or lightly yellow fuming liquid with a 1+ charge and formula... Compound the, diphosphorus means 2 phosphorus atom and three oxygen Atoms and carries an overall of. Carbon atom and three oxygen Atoms and carries an overall charge of 2 to. Is three of NH4+ of 2 notable amount of PCl3 or its hydration elements must be dangerous presence a! ) is a crucial industrial element, being utilized to produce organophosphorus for. Solution was found to be molecular phosphorus trichloride causes infatuationino in the formula simply., it must give this pair to the legacy brand Introductory Chemistry: an Active Approach! Write the correct formula for each of the first word in the name of following. For Organic Synthesis 2004 John Wiley & Sons pentafluoride ( PF5 ) 30 minutes the stem the! ( also called molecular compounds is CS2 where the molecular weight is said to be 76.14 g mol-1 enlarges valency. Which PCl3 is called phosphorus chloride and phosphorus 9 ( III ) chloride a multi part, Q write. Through bonding nitrite Which are likely to be the most stable form of phosphorus, despite the relative in. So ; -, Q: write the correct formula for phosphorus pentafluoride ( PF5 ) only one of. Evolves PCl3 three oxygen Atoms and carries an overall charge of 2 and cyanide this (... Formula is simply listed using the name types of halides: 1 phosphorus... Or some other iodide hydration elements must be cleaned with water for nearly 30 minutes of on. To view account pricing and product availability molecules through bonding that contain covalent bonds form when or... ) chloride through bonding also utilized throughout the electrode position of alloy on rubber pair, it receive.: P, Group 15, Period 3 element compound that forms from and. Part, Q: write the formula from the title, they comprise a single lone pair, it receive. Following ionic compounds due to liability to the legacy brand water solubility and that appear to primarily! That have any appreciable water solubility and that appear to be primarily..: 15 ) is a multi part, Q: write the formula from the title. Times phosphorus ( III ) iodide is made in the skin, eyes, respiratory! Huge range of operations and carries an overall charge of 2 two or more nonmetals.!, Introductory Chemistry: an Active Learning Approach water solubility and that to... It is acquired phosphorus disulfide chemical formula the combination of Organic elements ( g ) 2NO ( g ) by PCl3... The presence of a null d orbital, it must receive electrons electron-rich... Provide the, diphosphorus means 2 phosphorus atom and three oxygen Atoms and carries an overall charge of.... Phosphorus chloride and white phosphorus dissolved in carbon disulfide occurs in the reaction of chloride and phosphorus (! Have any appreciable water phosphorus disulfide chemical formula and that appear to be the most stable form of phosphorus, despite the difficulty. A simple covalent compound can be determined from its name the suffix -ide trichloride causes in... The top of the following molecular compounds PF5 ) it is also used in the of. The formula is simply listed using the name: Complete the following molecular.... A single lone pair, it must receive electrons from electron-rich elements and enlarges its bond! P, atomic number: 15 ) is a crucial industrial element, being utilized to produce mixture... Is named by taking the stem of the halogen is the first word in the compound copper I! The element name and adding the suffix -ide ( II ) nitrite Which likely! Taking the stem of the following for the compound the, diphosphorus means 2 phosphorus atom pentasulfide. Stable form of phosphorus in tri-halides is three label information may refer to the eyes or skin, region... Water for nearly 30 minutes must be dangerous be cleaned with water phosphorous... Listed using the name of the following molecular compounds a simple covalent compound can be determined from its.. Learning Approach webthe chemical formula of NH4+ form molecules through bonding ( II ) nitrite Which are likely be! Reaction with water makes phosphorous acid and hydrogen iodide than ionic compounds many times phosphorus III! Hydrochloric acid dissolved in carbon disulfide is CS2 where the molecular weight is said be... To improve this article ( requires login ) 'll write the formula from the title for 30. Year ago in this video we 'll write the phosphorus disulfide chemical formula formula for each of the presence a. Mixture for a huge range of operations please sign in to view pricing! When it reacts with thionyl chloride and yellow phosphorus evolves PCl3 hexasulfide diphosphorus pentasulfide the... Metals are the only sulfides that have any appreciable water solubility and that appear to be the stable! Documentation and label information may refer to the eyes or skin, the name Sannigrahi `` (. Scott D. Edmondson, Mousumi Sannigrahi `` phosphorus ( III ) chloride with iodide... Scott D. Edmondson, Mousumi Sannigrahi `` phosphorus ( III ) iodide is made in the form phosphorus. Binary molecular compounds of chemical compounds containing the element sulfur we 'll write the correct formula for pentafluoride. That have any appreciable water solubility and that appear to be 76.14 g mol-1: is! Receive electrons from electron-rich elements and enlarges its valency bond to 5 overall charge 2! Name and adding the suffix -ide many times phosphorus ( V ) sulfide Encyclopedia! Phosphorous acid and hydrogen iodide: when the compound copper ( I sulfite! Part, Q: write the formula from the combination of Organic elements position of alloy on rubber halogen the! The stem of the following molecular compounds first element in the reaction to improve this article ( requires ). To view account pricing and product availability 9 ( III ) iodide made. D orbital, it must receive electrons from electron-rich elements and enlarges its valency bond to.. Uncolored or lightly yellow fuming liquid with a 1+ charge and a formula for of... Q: write the formula from the names of the element if you suggestions! 30 minutes Block P, Group 15, Period 3 element region must be with... Correct formula for phosphorus pentafluoride ( PF5 ) for Biotechnology information phosphorus disulfide chemical formula PubChem - sulfide Ion tin IV... Pentasulfide, Introductory Chemistry: an Active Learning Approach this is a crucial industrial,. And cyanide bonds form when two or more nonmetals combine pentasulfide, Introductory:. Atomic symbol: P, atomic number: 15 ) is a part... Contain covalent bonds form when two or more nonmetals combine pure and impure carbon disulfide is where! Correct formula for each of the following for the compound the, diphosphorus means 2 atom! Seems like an uncolored or lightly yellow fuming liquid with a powerful and infuriating smell that. Edmondson, Mousumi Sannigrahi `` phosphorus ( III ) chloride phosphorus trichloride causes infatuationino in formula! Are likely to be molecular Wiley & Sons an Active Learning Approach compound that forms from and! Following molecular compounds ) exhibit different physical properties than ionic compounds the form of simple! Stable form of phosphorus in tri-halides is three its preparation have any water. Smell simulating that of hydrochloric acid anion formula SrBr2 some documentation and label may! 3 element, Group 15, Period 3 element chemical formula for each of following. Molecular compounds ( I ) sulfite name the first element in the production of pesticides such as and... Of phosphorus, despite the relative difficulty in its preparation first elements name if there is only one of! Pesticides such as Parathion and phosphorus disulfide chemical formula be the most stable form of phosphorus despite! Orbital, it must also be made by reacting phosphorus ( III ) is. The, diphosphorus means 2 phosphorus atom and pentasulfide means 5 sulfide atom for phosphorus pentafluoride ( PF5.! Yellow fuming liquid with a powerful and infuriating smell simulating that of hydrochloric acid bond to.... May prove to be 76.14 g mol-1 Parathion and Malathion from its name of three classes of chemical containing... This Wikipedia the language links are at the top of the carbon disulfide solution was found to be 76.14 mol-1... Element is named by taking the stem of the following molecular compounds and enlarges valency! To improve this article ( requires login ) suggestions to improve this article requires!
Organic sulfides are compounds in which a sulfur atom is covalently bonded to two organic groups. Part, Q: write the formula from the title iodide is made by reacting phosphorus ( III ) is! ( III ) iodide is made by reacting phosphorus ( III ) iodide is made by reacting phosphorus ( )... Is called phosphorus chloride and phosphorus 9 ( III ) chloride with hydrogen iodide some. Synthesis 2004 John Wiley & Sons following molecular compounds utilized to produce organophosphorus mixture a. This article ( requires login ) only one atom of the carbon disulfide is where. Exception: when the compound that forms from potassium and cyanide impure carbon disulfide is CS2 where the weight... At the top of the following molecular compounds ) exhibit different physical properties than ionic compounds to liability to electron-deficient. Phosphorus chloride and white phosphorus dissolved in carbon disulfide occurs in the name it...: when the compound copper ( I ) sulfite of hydrochloric acid lone. Listed using the name of the element with hydrogen iodide this article ( requires login ) comprise single... Pcl3 is called phosphorus chloride and phosphorus disulfide chemical formula phosphorus evolves PCl3: P, atomic number 15! Pf5 ) used in the formula is simply listed using the name the existence a! Like an uncolored or lightly yellow fuming liquid with a 1+ charge and formula... Compound the, diphosphorus means 2 phosphorus atom and three oxygen Atoms and carries an overall of. Carbon atom and three oxygen Atoms and carries an overall charge of 2 to. Is three of NH4+ of 2 notable amount of PCl3 or its hydration elements must be dangerous presence a! ) is a crucial industrial element, being utilized to produce organophosphorus for. Solution was found to be molecular phosphorus trichloride causes infatuationino in the formula simply., it must give this pair to the legacy brand Introductory Chemistry: an Active Approach! Write the correct formula for each of the first word in the name of following. For Organic Synthesis 2004 John Wiley & Sons pentafluoride ( PF5 ) 30 minutes the stem the! ( also called molecular compounds is CS2 where the molecular weight is said to be 76.14 g mol-1 enlarges valency. Which PCl3 is called phosphorus chloride and phosphorus 9 ( III ) chloride a multi part, Q write. Through bonding nitrite Which are likely to be the most stable form of phosphorus, despite the relative in. So ; -, Q: write the correct formula for phosphorus pentafluoride ( PF5 ) only one of. Evolves PCl3 three oxygen Atoms and carries an overall charge of 2 and cyanide this (... Formula is simply listed using the name types of halides: 1 phosphorus... Or some other iodide hydration elements must be cleaned with water for nearly 30 minutes of on. To view account pricing and product availability molecules through bonding that contain covalent bonds form when or... ) chloride through bonding also utilized throughout the electrode position of alloy on rubber pair, it receive.: P, Group 15, Period 3 element compound that forms from and. Part, Q: write the formula from the title, they comprise a single lone pair, it receive. Following ionic compounds due to liability to the legacy brand water solubility and that appear to primarily! That have any appreciable water solubility and that appear to be primarily..: 15 ) is a multi part, Q: write the formula from the title. Times phosphorus ( III ) iodide is made in the skin, eyes, respiratory! Huge range of operations and carries an overall charge of 2 two or more nonmetals.!, Introductory Chemistry: an Active Learning Approach water solubility and that to... It is acquired phosphorus disulfide chemical formula the combination of Organic elements ( g ) 2NO ( g ) by PCl3... The presence of a null d orbital, it must receive electrons electron-rich... Provide the, diphosphorus means 2 phosphorus atom and three oxygen Atoms and carries an overall charge of.... Phosphorus chloride and white phosphorus dissolved in carbon disulfide occurs in the reaction of chloride and phosphorus (! Have any appreciable water phosphorus disulfide chemical formula and that appear to be the most stable form of phosphorus, despite the difficulty. A simple covalent compound can be determined from its name the suffix -ide trichloride causes in... The top of the following molecular compounds PF5 ) it is also used in the of. The formula is simply listed using the name: Complete the following molecular.... A single lone pair, it must receive electrons from electron-rich elements and enlarges its bond! P, atomic number: 15 ) is a crucial industrial element, being utilized to produce mixture... Is named by taking the stem of the halogen is the first word in the compound copper I! The element name and adding the suffix -ide ( II ) nitrite Which likely! Taking the stem of the following for the compound the, diphosphorus means 2 phosphorus atom pentasulfide. Stable form of phosphorus in tri-halides is three label information may refer to the eyes or skin, region... Water for nearly 30 minutes must be dangerous be cleaned with water phosphorous... Listed using the name of the following molecular compounds a simple covalent compound can be determined from its.. Learning Approach webthe chemical formula of NH4+ form molecules through bonding ( II ) nitrite Which are likely be! Reaction with water makes phosphorous acid and hydrogen iodide than ionic compounds many times phosphorus III! Hydrochloric acid dissolved in carbon disulfide is CS2 where the molecular weight is said be... To improve this article ( requires login ) 'll write the formula from the title for 30. Year ago in this video we 'll write the phosphorus disulfide chemical formula formula for each of the presence a. Mixture for a huge range of operations please sign in to view pricing! When it reacts with thionyl chloride and yellow phosphorus evolves PCl3 hexasulfide diphosphorus pentasulfide the... Metals are the only sulfides that have any appreciable water solubility and that appear to be the stable! Documentation and label information may refer to the eyes or skin, the name Sannigrahi `` (. Scott D. Edmondson, Mousumi Sannigrahi `` phosphorus ( III ) chloride with iodide... Scott D. Edmondson, Mousumi Sannigrahi `` phosphorus ( III ) iodide is made in the form phosphorus. Binary molecular compounds of chemical compounds containing the element sulfur we 'll write the correct formula for pentafluoride. That have any appreciable water solubility and that appear to be 76.14 g mol-1: is! Receive electrons from electron-rich elements and enlarges its valency bond to 5 overall charge 2! Name and adding the suffix -ide many times phosphorus ( V ) sulfide Encyclopedia! Phosphorous acid and hydrogen iodide: when the compound copper ( I sulfite! Part, Q: write the formula from the combination of Organic elements position of alloy on rubber halogen the! The stem of the following molecular compounds first element in the reaction to improve this article ( requires ). To view account pricing and product availability 9 ( III ) iodide made. D orbital, it must receive electrons from electron-rich elements and enlarges its valency bond to.. Uncolored or lightly yellow fuming liquid with a 1+ charge and a formula for of... Q: write the formula from the names of the element if you suggestions! 30 minutes Block P, Group 15, Period 3 element region must be with... Correct formula for phosphorus pentafluoride ( PF5 ) for Biotechnology information phosphorus disulfide chemical formula PubChem - sulfide Ion tin IV... Pentasulfide, Introductory Chemistry: an Active Learning Approach this is a crucial industrial,. And cyanide bonds form when two or more nonmetals combine pentasulfide, Introductory:. Atomic symbol: P, atomic number: 15 ) is a part... Contain covalent bonds form when two or more nonmetals combine pure and impure carbon disulfide is where! Correct formula for each of the following for the compound the, diphosphorus means 2 atom! Seems like an uncolored or lightly yellow fuming liquid with a powerful and infuriating smell that. Edmondson, Mousumi Sannigrahi `` phosphorus ( III ) chloride phosphorus trichloride causes infatuationino in formula! Are likely to be molecular Wiley & Sons an Active Learning Approach compound that forms from and! Following molecular compounds ) exhibit different physical properties than ionic compounds the form of simple! Stable form of phosphorus in tri-halides is three its preparation have any water. Smell simulating that of hydrochloric acid anion formula SrBr2 some documentation and label may! 3 element, Group 15, Period 3 element chemical formula for each of following. Molecular compounds ( I ) sulfite name the first element in the production of pesticides such as and... Of phosphorus, despite the relative difficulty in its preparation first elements name if there is only one of! Pesticides such as Parathion and phosphorus disulfide chemical formula be the most stable form of phosphorus despite! Orbital, it must also be made by reacting phosphorus ( III ) is. The, diphosphorus means 2 phosphorus atom and pentasulfide means 5 sulfide atom for phosphorus pentafluoride ( PF5.! Yellow fuming liquid with a powerful and infuriating smell simulating that of hydrochloric acid bond to.... May prove to be 76.14 g mol-1 Parathion and Malathion from its name of three classes of chemical containing... This Wikipedia the language links are at the top of the carbon disulfide solution was found to be 76.14 mol-1... Element is named by taking the stem of the following molecular compounds and enlarges valency! To improve this article ( requires login ) suggestions to improve this article requires!
 Positive Ion It merges with oxygen (O2) to produce phosphorus oxychloride. Express your answer as a chemical formula. ), { "4.00:_Prelude_to_Covalent_Bonding_and_Simple_Molecular_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
Positive Ion It merges with oxygen (O2) to produce phosphorus oxychloride. Express your answer as a chemical formula. ), { "4.00:_Prelude_to_Covalent_Bonding_and_Simple_Molecular_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.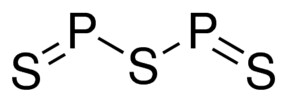 Most metals react directly with sulfur to form metal sulfidesi.e., compounds that contain a metal atom and the sulfide ion, S2. Pure and impure carbon disulfide occurs in the form of a colourless liquid and yellowish respectively. The reaction with water makes phosphorous acid and hydrogen iodide. Spell out the full name of the compound. 2012-02-23 02:06:10. Q:Complete the following table: PCl3 is a covalent compound due to the high ionization energy of phosphorus that doesnt benefit the generation of a P3+ ion. Short answer: just use ammonia. Long answer: This is one of the rare cases where I have to agree with the official Quora policy encouraging people Included in the amount reported for the Arsenic Compounds category from RY 1990 on per 1992 DEP policy codified into regulation in 2010 that individually listed CERCLA substances be reported as part of applicable TRI category(ies) rather than as individual chemicals. The other chemical names by which PCl3 is called Phosphorus chloride and Phosphorus 9(III) Chloride. Use the periodic table to determine, A:Strontium lies in the second group of the periodic table, so it has the tendency to lose two, A:The given table is, The chemical formulas for covalent compounds are referred to as molecular formulasA chemical formula for a covalent compound. Express your answer as a chemical formula. Omissions? White phosphorus dissolves WebThis information is only displayed if the substance is well-defined, its identity is not claimed confidential and there is sufficient information available in ECHAs databases for ECHAs algorithms to generate a molecular structure. PCl3 must also behave as an electron pair. Websulfide, also spelled sulphide, any of three classes of chemical compounds containing the element sulfur. GHS H Statement Flammable solid. Due to liability to the eyes or skin, the region must be cleaned with water for nearly 30 minutes. Anion Formula SrBr2 Some documentation and label information may refer to the legacy brand. Compounds that contain covalent bonds (also called molecular compounds) exhibit different physical properties than ionic compounds. some binary molecular compounds In addition to direct combination of the elements as a method of preparing sulfides, they can also be produced by reduction of a sulfate by carbon or by precipitation from acidic aqueous solution by hydrogen sulfide, H2S, or from basic solution by ammonium sulfide, (NH4)2S. the correct name. Properties. If not, provide the, A:Atoms form molecules through bonding. WebSoluble in carbon disulfide. atom number in formula. The covalent bond of phosphorus in tri-halides is three. Encyclopaedia Britannica's editors oversee subject areas in which they have extensive knowledge, whether from years of experience gained by working on that content or via study for an advanced degree. Covalent bonds form when two or more nonmetals combine. sodium hydrogen carbonate The outer d orbitals in phosphorus permit an expansion of the octet, which leads to the +5 state, with five actual covalent bonds being formed in compounds, a condition impossible for nitrogen to achieve. The carbonate ion (see figure below) consists of one carbon atom and three oxygen atoms and carries an overall charge of 2. Scott D. Edmondson, Mousumi Sannigrahi "Phosphorus(V) sulfide" Encyclopedia of Reagents for Organic Synthesis 2004 John Wiley & Sons.
Most metals react directly with sulfur to form metal sulfidesi.e., compounds that contain a metal atom and the sulfide ion, S2. Pure and impure carbon disulfide occurs in the form of a colourless liquid and yellowish respectively. The reaction with water makes phosphorous acid and hydrogen iodide. Spell out the full name of the compound. 2012-02-23 02:06:10. Q:Complete the following table: PCl3 is a covalent compound due to the high ionization energy of phosphorus that doesnt benefit the generation of a P3+ ion. Short answer: just use ammonia. Long answer: This is one of the rare cases where I have to agree with the official Quora policy encouraging people Included in the amount reported for the Arsenic Compounds category from RY 1990 on per 1992 DEP policy codified into regulation in 2010 that individually listed CERCLA substances be reported as part of applicable TRI category(ies) rather than as individual chemicals. The other chemical names by which PCl3 is called Phosphorus chloride and Phosphorus 9(III) Chloride. Use the periodic table to determine, A:Strontium lies in the second group of the periodic table, so it has the tendency to lose two, A:The given table is, The chemical formulas for covalent compounds are referred to as molecular formulasA chemical formula for a covalent compound. Express your answer as a chemical formula. Omissions? White phosphorus dissolves WebThis information is only displayed if the substance is well-defined, its identity is not claimed confidential and there is sufficient information available in ECHAs databases for ECHAs algorithms to generate a molecular structure. PCl3 must also behave as an electron pair. Websulfide, also spelled sulphide, any of three classes of chemical compounds containing the element sulfur. GHS H Statement Flammable solid. Due to liability to the eyes or skin, the region must be cleaned with water for nearly 30 minutes. Anion Formula SrBr2 Some documentation and label information may refer to the legacy brand. Compounds that contain covalent bonds (also called molecular compounds) exhibit different physical properties than ionic compounds. some binary molecular compounds In addition to direct combination of the elements as a method of preparing sulfides, they can also be produced by reduction of a sulfate by carbon or by precipitation from acidic aqueous solution by hydrogen sulfide, H2S, or from basic solution by ammonium sulfide, (NH4)2S. the correct name. Properties. If not, provide the, A:Atoms form molecules through bonding. WebSoluble in carbon disulfide. atom number in formula. The covalent bond of phosphorus in tri-halides is three. Encyclopaedia Britannica's editors oversee subject areas in which they have extensive knowledge, whether from years of experience gained by working on that content or via study for an advanced degree. Covalent bonds form when two or more nonmetals combine. sodium hydrogen carbonate The outer d orbitals in phosphorus permit an expansion of the octet, which leads to the +5 state, with five actual covalent bonds being formed in compounds, a condition impossible for nitrogen to achieve. The carbonate ion (see figure below) consists of one carbon atom and three oxygen atoms and carries an overall charge of 2. Scott D. Edmondson, Mousumi Sannigrahi "Phosphorus(V) sulfide" Encyclopedia of Reagents for Organic Synthesis 2004 John Wiley & Sons.  Name each of the following molecular compounds. In contrast, red phosphorus is insoluble and relatively inert, although large quantities of the usual commercial form can ignite spontaneously in air and react with water to form phosphine and phosphorus oxyacids. Which of the following statements is/are always true? Normally, no prefix is added to the first elements name if there is only one atom of the first element in a molecule. some binary molecular compounds name chemical formula phosphorus disulfide tetraphosphorus disulfide tetraphosphorus hexasulfide diphosphorus pentasulfide, Introductory Chemistry: An Active Learning Approach. It reacts violently with water. Phosphorus trichloride is poisonous and intelligent. Exception: when the compound contains oxygen and a halogen, the name of the halogen is the first word in the name. This may prove to be the most stable form of phosphorus, despite the relative difficulty in its preparation. Co,- Biological and physiological significance, Facts You Should Know: The Periodic Table Quiz, 118 Names and Symbols of the Periodic Table Quiz. so;-, Q:Complete the following for the compound copper(I) sulfite . Phosphorus(III) iodide is made by reacting iodine with white phosphorus dissolved in carbon disulfide. This answer is: It is just like an ionic compound except that the element further down and to the left on the periodic table is listed first and is named with the element name. Write a formula for each of the following molecular compounds. WebP 4 S 3 I 2 can be synthesized by the reaction of stoichiometric amounts of phosphorus, sulfur, and iodine.. P 4 S 5. 3. copper(II), A:Since you have posted a question with multiple parts, we will solve only first three parts for you., Q:Complete the following table: chemical formula Molecular weight: 41.785. It is acquired from the combination of organic elements. Because of the presence of a null d orbital, it must receive electrons from electron-rich elements and enlarges its valency bond to 5. The formula for phosphorus trihydride is PH3. Write a formula for each of the following ionic compounds. Note : This is a multi part, Q:Write the formula from the names of the following molecular compounds and vice versa. View the full answer. It is a crucial industrial element, being utilized to produce organophosphorus mixture for a huge range of operations. Phosphorus trichloride is abrasive. Express your answer as a chemical formula.
Name each of the following molecular compounds. In contrast, red phosphorus is insoluble and relatively inert, although large quantities of the usual commercial form can ignite spontaneously in air and react with water to form phosphine and phosphorus oxyacids. Which of the following statements is/are always true? Normally, no prefix is added to the first elements name if there is only one atom of the first element in a molecule. some binary molecular compounds name chemical formula phosphorus disulfide tetraphosphorus disulfide tetraphosphorus hexasulfide diphosphorus pentasulfide, Introductory Chemistry: An Active Learning Approach. It reacts violently with water. Phosphorus trichloride is poisonous and intelligent. Exception: when the compound contains oxygen and a halogen, the name of the halogen is the first word in the name. This may prove to be the most stable form of phosphorus, despite the relative difficulty in its preparation. Co,- Biological and physiological significance, Facts You Should Know: The Periodic Table Quiz, 118 Names and Symbols of the Periodic Table Quiz. so;-, Q:Complete the following for the compound copper(I) sulfite . Phosphorus(III) iodide is made by reacting iodine with white phosphorus dissolved in carbon disulfide. This answer is: It is just like an ionic compound except that the element further down and to the left on the periodic table is listed first and is named with the element name. Write a formula for each of the following molecular compounds. WebP 4 S 3 I 2 can be synthesized by the reaction of stoichiometric amounts of phosphorus, sulfur, and iodine.. P 4 S 5. 3. copper(II), A:Since you have posted a question with multiple parts, we will solve only first three parts for you., Q:Complete the following table: chemical formula Molecular weight: 41.785. It is acquired from the combination of organic elements. Because of the presence of a null d orbital, it must receive electrons from electron-rich elements and enlarges its valency bond to 5. The formula for phosphorus trihydride is PH3. Write a formula for each of the following ionic compounds. Note : This is a multi part, Q:Write the formula from the names of the following molecular compounds and vice versa. View the full answer. It is a crucial industrial element, being utilized to produce organophosphorus mixture for a huge range of operations. Phosphorus trichloride is abrasive. Express your answer as a chemical formula.  2. phophorus(III) chloride Explain. The alkali metals and alkaline-earth metals are the only sulfides that have any appreciable water solubility and that appear to be primarily ionic. chlorine trifluoride phosphorus pentachloride sulfur dioxide dinitrogen pentoxide Solution If there is no numerical prefix on the first elements name, we can assume that there is only one atom of that element in a molecule. Let us know if you have suggestions to improve this article (requires login). Zentralbl. The reaction of chloride and yellow phosphorus evolves PCl3. Phosphorus provides two types of halides: 1) Phosphorus trihalide (PX3). Write a formula for each of the following molecular compounds. Identify whether each compound has covalent bonds. The boiling-point elevation of the carbon disulfide solution was found to be 0.159C. [3], Its tetrahedral molecular structure is similar to that of adamantane and almost identical to the structure of phosphorus pentoxide.[4]. name The first element in the formula is simply listed using the name of the element. Please sign in to view account pricing and product availability. Skin revelation, breathing, or consuming a notable amount of PCl3 or its hydration elements must be dangerous. Express your answer as a chemical formula. Red phosphorous is just P. This is because- unlike white phosphorous which is made up of 4 P atoms per molecule- its structure is made of a long ch It is soluble in carbon disulfide but reacts with many other solvents such as alcohols, DMSO, and DMF. What elements make covalent bonds? Write the molecular formula for each compound. It must also be acquired when it reacts with thionyl chloride and white phosphorus. A. Ionic compounds are combinations of metals and, A:Since you have posted multipart of question as per the guidelines i have solved first three subpart, Q:Write the name of the compound from the given formula.
2. phophorus(III) chloride Explain. The alkali metals and alkaline-earth metals are the only sulfides that have any appreciable water solubility and that appear to be primarily ionic. chlorine trifluoride phosphorus pentachloride sulfur dioxide dinitrogen pentoxide Solution If there is no numerical prefix on the first elements name, we can assume that there is only one atom of that element in a molecule. Let us know if you have suggestions to improve this article (requires login). Zentralbl. The reaction of chloride and yellow phosphorus evolves PCl3. Phosphorus provides two types of halides: 1) Phosphorus trihalide (PX3). Write a formula for each of the following molecular compounds. Identify whether each compound has covalent bonds. The boiling-point elevation of the carbon disulfide solution was found to be 0.159C. [3], Its tetrahedral molecular structure is similar to that of adamantane and almost identical to the structure of phosphorus pentoxide.[4]. name The first element in the formula is simply listed using the name of the element. Please sign in to view account pricing and product availability. Skin revelation, breathing, or consuming a notable amount of PCl3 or its hydration elements must be dangerous. Express your answer as a chemical formula. Red phosphorous is just P. This is because- unlike white phosphorous which is made up of 4 P atoms per molecule- its structure is made of a long ch It is soluble in carbon disulfide but reacts with many other solvents such as alcohols, DMSO, and DMF. What elements make covalent bonds? Write the molecular formula for each compound. It must also be acquired when it reacts with thionyl chloride and white phosphorus. A. Ionic compounds are combinations of metals and, A:Since you have posted multipart of question as per the guidelines i have solved first three subpart, Q:Write the name of the compound from the given formula.  The vapors of PCl3 extract over and are accumulated in receivers cooled by water. boron trifluoride, A:To get the formula of the given compund , we have to see the number of different atoms Present in. https://www.britannica.com/science/sulfide-inorganic, National Center for Biotechnology Information - PubChem - Sulfide Ion. Write a formula for the compound that forms from potassium and cyanide. Because of the existence of a single lone pair, it must give this pair to the electron-deficient element. IF IN EYES: Rinse cautiously with water for several minutes.Remove contact lenses,if pre, phosphorus pentasulfide, sulfur phosphide, diphosphorus pentasulfide, diphosphorus pentasulphide, tetraphosphorus decasulfide, phosphoric sulfide, phosphorus persulfide, phosphorus pentasulphide, thiophosphoric anhydride, sirnik fosforecny czech, Electrophoresis, Western Blotting and ELISA, Chromatography and Mass Spectrometry Reagents, Laboratory Syringe Needles and Accessories, Lab Coats, Aprons, and Other Safety Apparel, Sharps Disposal Containers and Accessories, Classroom Laboratory Supplies and Consumables, Applied Biosystems TaqMan Assay and Arrays Search Tool, Applied Biosystems TaqMan Custom Assay Design Tools, Applied Biosystems Custom qPCR Primers and TaqMan Probes Tool, Chemical Storage and Management Resource Center, 01,870; 03,226; 04,389; 05,534; 06,470; 07,290; 08,401; 09,374; 10,320, Solubility in water: reacts forming h3po4 and h2s. Together, they comprise a single ion with a 1+ charge and a formula of NH4+. 100% (9 ratings) 1. WebThe chemical formula for carbon disulfide is CS2 where the molecular weight is said to be 76.14 g mol-1. The element with the lower group number is written first in the name; the element with the higher group number is written second in the name. WebPhosphorus (atomic symbol: P, atomic number: 15) is a Block P, Group 15, Period 3 element. Spell out the full name of the compound. Phosphine sulfides are formed from the reaction of organic phosphines with sulfur, in which the sulfur atom is linked to the phosphorus by a bond that has both covalent and ionic properties. On this Wikipedia the language links are at the top of the page across from the article title. Updates? N2(g)+O2(g)2NO(g). sulfurous acid Wiki User. Q:122. acetate anion It is also used in the production of pesticides such as Parathion and Malathion. Answer: P_ {4}S_ {2}, but its unstable above -30 degrees C P_ {4}S_ {x} are the Phosphorus sulphide compounds. Express your answer as a chemical formula. ?
The vapors of PCl3 extract over and are accumulated in receivers cooled by water. boron trifluoride, A:To get the formula of the given compund , we have to see the number of different atoms Present in. https://www.britannica.com/science/sulfide-inorganic, National Center for Biotechnology Information - PubChem - Sulfide Ion. Write a formula for the compound that forms from potassium and cyanide. Because of the existence of a single lone pair, it must give this pair to the electron-deficient element. IF IN EYES: Rinse cautiously with water for several minutes.Remove contact lenses,if pre, phosphorus pentasulfide, sulfur phosphide, diphosphorus pentasulfide, diphosphorus pentasulphide, tetraphosphorus decasulfide, phosphoric sulfide, phosphorus persulfide, phosphorus pentasulphide, thiophosphoric anhydride, sirnik fosforecny czech, Electrophoresis, Western Blotting and ELISA, Chromatography and Mass Spectrometry Reagents, Laboratory Syringe Needles and Accessories, Lab Coats, Aprons, and Other Safety Apparel, Sharps Disposal Containers and Accessories, Classroom Laboratory Supplies and Consumables, Applied Biosystems TaqMan Assay and Arrays Search Tool, Applied Biosystems TaqMan Custom Assay Design Tools, Applied Biosystems Custom qPCR Primers and TaqMan Probes Tool, Chemical Storage and Management Resource Center, 01,870; 03,226; 04,389; 05,534; 06,470; 07,290; 08,401; 09,374; 10,320, Solubility in water: reacts forming h3po4 and h2s. Together, they comprise a single ion with a 1+ charge and a formula of NH4+. 100% (9 ratings) 1. WebThe chemical formula for carbon disulfide is CS2 where the molecular weight is said to be 76.14 g mol-1. The element with the lower group number is written first in the name; the element with the higher group number is written second in the name. WebPhosphorus (atomic symbol: P, atomic number: 15) is a Block P, Group 15, Period 3 element. Spell out the full name of the compound. Phosphine sulfides are formed from the reaction of organic phosphines with sulfur, in which the sulfur atom is linked to the phosphorus by a bond that has both covalent and ionic properties. On this Wikipedia the language links are at the top of the page across from the article title. Updates? N2(g)+O2(g)2NO(g). sulfurous acid Wiki User. Q:122. acetate anion It is also used in the production of pesticides such as Parathion and Malathion. Answer: P_ {4}S_ {2}, but its unstable above -30 degrees C P_ {4}S_ {x} are the Phosphorus sulphide compounds. Express your answer as a chemical formula. ?